
 |
ROLEXROLEXROLEXROLEXROLEXROLEX
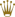 ROLEXROLEXROLEXROLEXROLEXROLEX
ROLEXROLEXROLEXROLEXROLEXROLEX
|
|
#1 |
|
"TRF" Member
Join Date: Mar 2007
Location: Margate
Watch: your back
Posts: 324
|
904L steel better?, maybe a myth
I don't remember who it was that wrote about 904L being better against corrosion in salt water and more acidic conditions being just a Rolex advertising bragging slogan. I looked all over these threads but cannot find who it was. If this metal has been used since the introduction of the 3135 mvt, then he may as well be right. Just take a look at the case corrosion on the back of this midcase date 15200 of this site;
http://www.interwatches.com/rolex-da...-132251-bg.jpg   I haven't posted it as an image as I do not own the rights to it, but one thing is for sure, at least this seller is honest. |
|
|

|
|
|
#2 |
|
"TRF" Life Patron
Join Date: Jun 2005
Real Name: Peter
Location: Llanfairpwllgwyng
Watch: ing you.
Posts: 53,062
|
Its quite simple as long as any watch is rinsed in fresh water after exposure to salt water and kept clean in its life.The watch will last for decades no matter what the S.steel while the 904L is slightly more resistant to corrosive substances.Just look at all the vintage Rolex for one in this world today.Plus the millions millions of watches with 316L the industry norm.I did have a old Rolex Unicorn from the 1920s one of the first watches to use S.steel for the case and in those days it was far more expensive than the silver version.Now that case for in excellent condition for its age.And lets be honest here today most Rolex watches get a very very pampered life.So those 95% of pampered Rolex watches would make no difference what ever the S.steel case was made from.IMHO the use of 904L is little more today than a pure Rolex brag and just pure marketing.And would hardly call a bit of o-ring residue and build up in the o-ring grove corrosion.Now its possible that watch was not serviced for a very long time,and all that would have been cleaned and made good as new as per service.
__________________
 ICom Pro3 All posts are my own opinion and my opinion only. "The clock of life is wound but once, and no man has the power to tell just when the hands will stop. Now is the only time you actually own the time, Place no faith in time, for the clock may soon be still for ever." Good Judgement comes from experience,experience comes from Bad Judgement,.Buy quality, cry once; buy cheap, cry again and again. www.mc0yad.club Second in command CEO and left handed watch winder 
|
|
|

|
|
|
#3 | |
|
"TRF" Member
Join Date: Mar 2007
Location: Margate
Watch: your back
Posts: 324
|
Quote:

|
|
|
|

|
|
|
#4 |
|
"TRF" Member
Join Date: Feb 2009
Real Name: Nat Parkinson
Location: Maine, USA
Watch: me pull a rabbit..
Posts: 1,196
|
Electrolysis
Actually I think the watch in your link just has some old degraded gasket material - not corrosion or pitting.
Here is what actual pitting looks like - this is said to be a 16013 from the mid '80s but I can't verify - web shot - 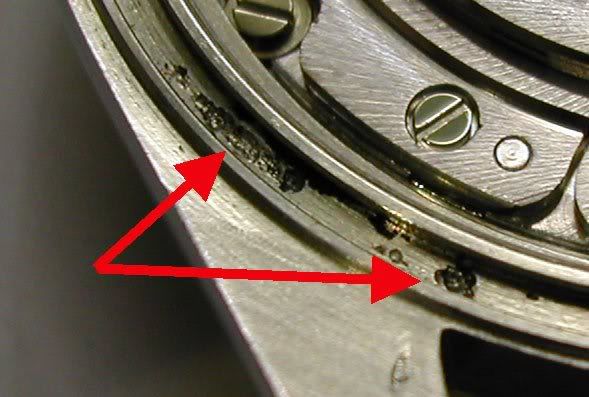 Sweat contains salt. (that's why you eat saline tablets when involved in sports or jungle combat) Salt water is the ideal conductive medium for electrolysis - on ships and boats, zinc plates are affixed to the hull or around the prop shaft to draw this corrosive effect to a non essential area and spare more critical parts (like the screws holding your boat together  ) from melting away to mush. The zinc acts in the same way a lightning rod channels a lightning strike around a barn and grounds it into the earth. ) from melting away to mush. The zinc acts in the same way a lightning rod channels a lightning strike around a barn and grounds it into the earth.I lived on sailboats for years and constantly battled salt water corrosion. With modern fiberglass boats the only major concerns are prop shafts and thru hull fittings - but believe me having a 2 inch thru hull corrode and fail when you are 100 miles offshore is NOT FUN! (Picture a 2 inch fire hose pumping the entire Atlantic ocean straight up into your house at 1000 PSI) I always kept 2 wooden plugs wired to every thru hull fitting and a large mallet mounted on a bulkhead where it could be grabbed at 3 AM while panicking. The real problem with electrolysis comes when you add the element of disparate metals (steel, bronze, copper all together), salt water, and soaked wood. I spent 2 years trading up and down the Chesapeake Bay on this lovely 1940 Hinckley Islander. At the time it was nearly 50 years old.  Old wooden boats are fastened with "siliconized bronze" screws - which are reset and covered with wooden plugs. They have a remarkable resistance to corrosion, but nothing lasts 50 years in that environment. I once considered refastening the whole beast from stem to stern, until I found out that ONE screw cost $4. Sadly this beautiful boat was the victim of a hard freeze in 1996 - the whole Chesapeake froze solid and this boat sank like a rock after a couple of planks were stove in below the waterline.  All that remains is the maker's plate and memories. All that remains is the maker's plate and memories. My youngest daughter is actually named after this boat. 
__________________
When the alien spaceships actually landed, to everyone's surprise, it turned out that the world's governments had not been hiding anything. They were just as clueless as the rest of us! 
|
|
|

|
|
|
#5 |
|
"TRF" Member
Join Date: Mar 2007
Location: Margate
Watch: your back
Posts: 324
|
If you look closely to the top left hand side of the case just above the crown, you will notice some ridges of corrosion just over the top lip of the gasket groove. I am confident that it was more likely to be caused by sweat than sea salt or possibly a combination of both. The rest of the image can be degraded gasket material, but for a metal such as 904L steel which is supposedly meant to solve this problem makes me agree with Padi about the marketing factor by Rolex. He is right that most Rolex watches are over pampered by their owners as not everybody can afford to spend $thousands too often to purchase a new one. I had to wait over 20 years to get mine. I have had a couple Omega seamasters in highschool that belonged to my dad, never having had this corrosion problem and I did swim with them in the sea but never took too much notice on rinsing them in fresh water.
|
|
|

|
|
|
#6 |
|
"TRF" Life Patron
Join Date: Jun 2005
Real Name: Peter
Location: Llanfairpwllgwyng
Watch: ing you.
Posts: 53,062
|
If any watch is used often in a harsh environment like the sea then they should be pressure checked annually.And if necessary the seals renewed regularly regardless of the s.steel used.I have used both Rolex Vintage and modern plus my trusty Citizen Aqua-land 11 many years world wide underwater, and all have not had any problem with any corrosion whatsoever but all my dive watches serviced well.
__________________
 ICom Pro3 All posts are my own opinion and my opinion only. "The clock of life is wound but once, and no man has the power to tell just when the hands will stop. Now is the only time you actually own the time, Place no faith in time, for the clock may soon be still for ever." Good Judgement comes from experience,experience comes from Bad Judgement,.Buy quality, cry once; buy cheap, cry again and again. www.mc0yad.club Second in command CEO and left handed watch winder 
Last edited by padi56; 20 January 2010 at 03:49 AM.. |
|
|

|
|
|
#7 |
|
"TRF" Member
Join Date: Feb 2009
Real Name: Nat Parkinson
Location: Maine, USA
Watch: me pull a rabbit..
Posts: 1,196
|
I haven't posted it as an image as I do not own the rights to it, but one thing is for sure, at least this seller is honest.
I think it's OK to post a public image - what's not OK is using someone elses picture and implying that it is yours. As long as you say, like I did, "here's a picture I found from..." then you can post. I see what you mean. That's a drag...I guess the only preventatives would be to rinse your watch after any episode of sweating heavily - or - Affix a nice marine zinc to the back to bypass the steel with the softer zinc? Here's a good picture of what your watch would look like...nobody would notice. 
__________________
When the alien spaceships actually landed, to everyone's surprise, it turned out that the world's governments had not been hiding anything. They were just as clueless as the rest of us! 
|
|
|

|
|
|
#8 |
|
TRF Moderator & 2024 SubLV41 Patron
Join Date: May 2007
Real Name: Larry
Location: Mojave Desert
Watch: GMT's
Posts: 43,514
|
904L is not impervious, just more resistant to acids and corrosion - that's a fact. But it will still pit and corrode given the right circumstance...
The issue is the build up of corrosives under and around the gasket and seals.. Even a good rinse will not get rid of all of this.... That is a good reason why your watch should be serviced, cleaned and oiled at regular intervals.. Galvanic action and acid pitting doesn't happen overnight...it takes time... But it will happen more quickly in hot humid environments than more temperate ones..
__________________
(Chill ... It's just a watch Forum.....) NAWCC Member |
|
|

|
|
|
#9 |
|
"TRF" Member
Join Date: Dec 2007
Real Name: David
Location: USA
Watch: your step!
Posts: 7,882
|
My 304 SS license plate frames are subjected to awful winter sand/salt brine every year, and they are still holding up very well - there are pits from sand, but no corrosion. This is the front plate frame after 3 winters:
__________________
Rolex. The Rolex of watches. 16570 Expy2 Noir, 116710 GMT Master II, 2552.80 SMP |
|
|

|
|
|
#10 |
|
"TRF" Member
Join Date: Feb 2009
Real Name: Nat Parkinson
Location: Maine, USA
Watch: me pull a rabbit..
Posts: 1,196
|
Cool reflection of your EXP bracelet in that shot!
__________________
When the alien spaceships actually landed, to everyone's surprise, it turned out that the world's governments had not been hiding anything. They were just as clueless as the rest of us! 
|
|
|

|
|
|
#11 |
|
TRF Moderator & 2024 SubLV41 Patron
Join Date: May 2007
Real Name: Larry
Location: Mojave Desert
Watch: GMT's
Posts: 43,514
|
 They should make watches from license plate frames.... 
__________________
(Chill ... It's just a watch Forum.....) NAWCC Member |
|
|

|
|
|
#12 |
|
"TRF" Member
Join Date: Feb 2009
Real Name: Nat Parkinson
Location: Maine, USA
Watch: me pull a rabbit..
Posts: 1,196
|
They do...
They're called "Invicta's" 
__________________
When the alien spaceships actually landed, to everyone's surprise, it turned out that the world's governments had not been hiding anything. They were just as clueless as the rest of us! 
|
|
|

|
|
|
#13 |
|
Banned
Join Date: Dec 2007
Location: TX
Watch: Rolex Yacht-Master
Posts: 149
|
904L is more resistant to corrosion...but aren't they more susceptible to people with metal/nickel allergy?? I read that somewhere...i can't find it anymore.
|
|
|

|
|
|
#14 |
|
Fondly Remembered
Join Date: May 2005
Real Name: JJ
Location: Auckland, NZ
Watch: ALL SOLD!!
Posts: 74,319
|
  
__________________
Words fail me in expressing my utmost thanks to ALL of you for this wonderful support during my hour of need!!  I firmly believe that my time on planet earth is NOT yet up!! I shall fight this to the very end.......and WIN!! |
|
|

|
|
|
#15 | |
|
TRF Moderator & 2024 SubLV41 Patron
Join Date: May 2007
Real Name: Larry
Location: Mojave Desert
Watch: GMT's
Posts: 43,514
|
Quote:

__________________
(Chill ... It's just a watch Forum.....) NAWCC Member |
|
|
|

|
|
|
#16 |
|
"TRF" Member
Join Date: Dec 2007
Real Name: David
Location: USA
Watch: your step!
Posts: 7,882
|
Thanks buddy, here's another one using my excellent Microsoft Paint talents:
__________________
Rolex. The Rolex of watches. 16570 Expy2 Noir, 116710 GMT Master II, 2552.80 SMP |
|
|

|
|
|
#17 |
|
"TRF" Member
Join Date: Feb 2009
Real Name: Nat Parkinson
Location: Maine, USA
Watch: me pull a rabbit..
Posts: 1,196
|
Just checked - this is available here...Hmmmm

__________________
When the alien spaceships actually landed, to everyone's surprise, it turned out that the world's governments had not been hiding anything. They were just as clueless as the rest of us! 
|
|
|

|
|
|
#18 |
|
"TRF" Member
Join Date: Jul 2007
Real Name: Gary
Location: GMT-6
Watch: GMT
Posts: 3,350
|
Instead of worrying about nickel I wish Rolex would used hardened SS.
__________________
Omega Seamaster 300M GMT Noire Omega Seamaster Aqua Terra 8500 Benson 1937 Sterling Silver Hunter |
|
|

|
|
|
#19 |
|
Banned
Join Date: Oct 2008
Real Name: Hermann
Location: Middle of Europe
Watch: 16710/3185 16613 B
Posts: 413
|
|
|
|

|
|
|
#20 | |
|
Fondly Remembered
Join Date: May 2005
Real Name: JJ
Location: Auckland, NZ
Watch: ALL SOLD!!
Posts: 74,319
|
Quote:
 YIKES!! I thought I was seeing "wavy" with my right eye......then tried my left eye and it's still wavy!! YIKES!! I thought I was seeing "wavy" with my right eye......then tried my left eye and it's still wavy!!   
__________________
Words fail me in expressing my utmost thanks to ALL of you for this wonderful support during my hour of need!!  I firmly believe that my time on planet earth is NOT yet up!! I shall fight this to the very end.......and WIN!! |
|
|
|

|
|
|
#21 | |
|
"TRF" Member
Join Date: Feb 2009
Real Name: Nat Parkinson
Location: Maine, USA
Watch: me pull a rabbit..
Posts: 1,196
|
Quote:
The next night our 2 cops knocked on the door to return all the stolen presents -AFTER their wives had carefully rewrapped all of them.  Up here (and Maine has more vanity plates per capita than any other state in the US) it wouldn't get a second glance. You have the choice of 6 different plate themes. The other 5 "Rolex" plates are already in use. I have "Aflac" as a plate - and so do 3 other agents... Drives the cops nuts. 
__________________
When the alien spaceships actually landed, to everyone's surprise, it turned out that the world's governments had not been hiding anything. They were just as clueless as the rest of us! 
|
|
|
|

|
|
|
#22 |
|
"TRF" Member
Join Date: Apr 2008
Location: Australia
Posts: 95
|
Think Ive seen a similar pic somewhere on the net.
However, Rolex did't use 904L untill late 80's From what I can remember, the watch on the pic is 316L. Here I borrowed a pic from a TRF member, and this is the result after exposing 316 and 904 in salt water (maybe other test as well) 
|
|
|

|
 |
| Currently Active Users Viewing This Thread: 1 (0 members and 1 guests) | |
|
|
*Banners
Of The Month*
This space is provided to horological resources.